Cell Therapy Research and Manufacturing
Tumor-Directed T-cells Loaded with IL15 (RPTR147)
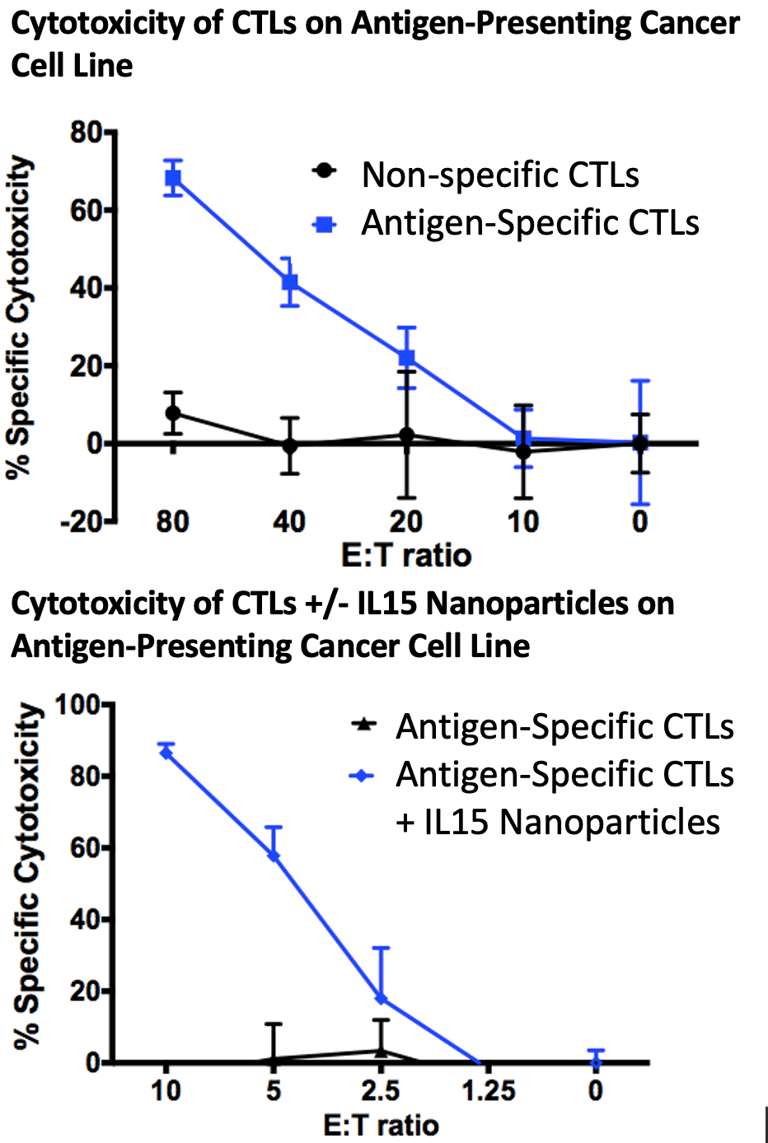
Torque used peripheral T-cells isolated from cancer patients as a starting point in the enrichment of tumor-reactive T-cells. The peripheral cells were co-cultured with matured dendritic cells loaded with tumor antigen. After expansion, the cells were loaded with an IL-15 nanoparticle and frozen as the drug product. The Cell Discovery and Analytic Development team built a comprehensive set of assays to describe the mechanism of action and find critical product parameters. In one assay, below, cells enriched with tumor antigen were incubated with a target cell line (left) in an in vitro cytotoxicity assay with non-specific T-cells used as a negative control demonstrating the specificity and cytotoxicity of the T-cells. Cells loaded with IL-15 nanoparticles had increased expansion and persistence and so were more cytotoxic at lower dilutions of effector to target (right).
Materials and CDMO Management for B-cell Therapy
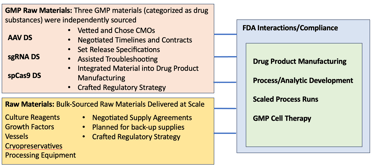
Walking Fish built and implemented a completely novel and scalable process and analytic development infrastructure for manufacturing a gene-edited B-cell therapy. Patient-derived B-cells were edited via CRISPR followed by transfection with an AAV-mediated transgene. The process consisted of four GMP materials manufactured at four sites, three drug-substances, and one drug product. Additionally, raw materials, some customized, had to be qualified for use in the process. All of these aspects came together in an FDA-compliant manner to support a B-cell process that generated as many as 20B gene-edited cells. In addition to the nine people driving process and analytic development at the Walking Fish site, we had an additional 75-people across all the CRO and CMO sites that were part of this complex undertaking.
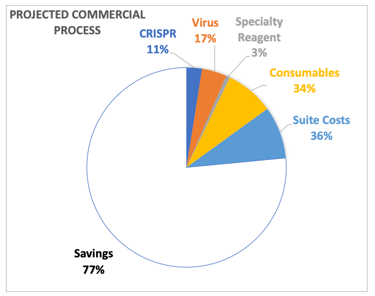
Cost of Goods and Life Cycle Management
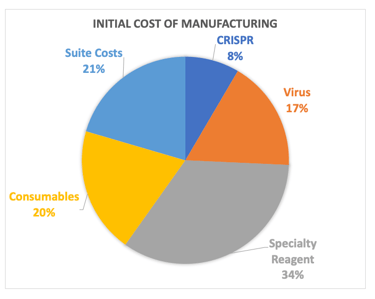
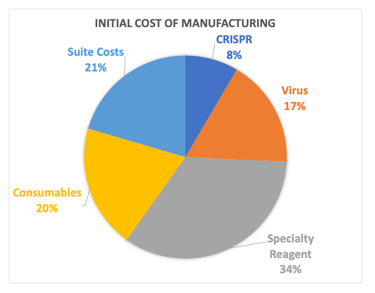
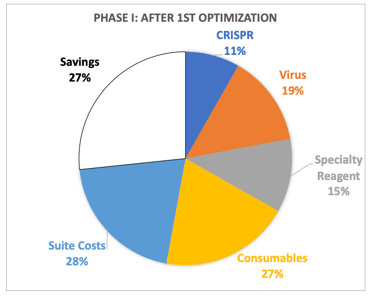
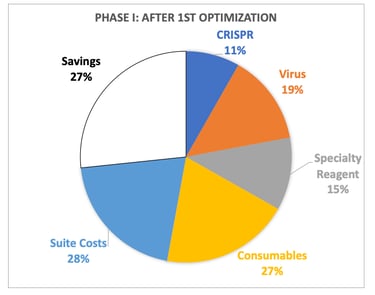
Cell Therapy Manufacturing is expensive and can be significant enough to sink a program, and COGS reduction is important. The initial B-cell process cost was dominated by consumables (mostly cytokines) and a specialty reagent needed for B-cell expansion, so we first optimized concentration and vendor selection for these things by which we got a 27% cost reduction. Ultimately, the team had planned on engineering and manufacturing its own specialty reagent which could be done much cheaper through a CMO. We were also going to gain efficiencies of scale in suite and virus costs which would have led to a 77% reduction in savings.